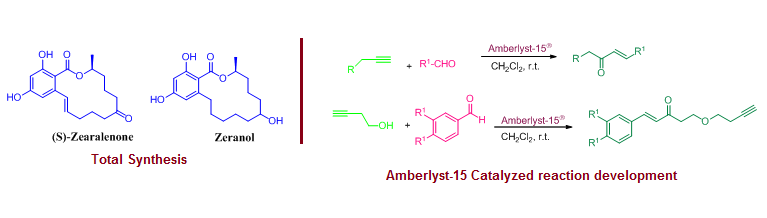
Total synthesis of (S)-Zearalenone and Zeranol and development of Amberlyst-15® promoted reactions
Abstract
This work review is structured in three different parts. The first part includes total synthesis of 14-membered macrolides (S)-zearalenone and zeralenone through the application of Diels-Alder reaction, Jacobsen kinetic resolution, Mitsunobu coupling, Ring-closing metathesis, and hydrogenation as key steps.  The second part discusses a convenient and metal-free protocol for the preparation of α,β-unsaturated ketones from alkynes and aldehydes using Amberlyst-15®. The third part describes the preparation of β-butynyloxy enones through the 2:1 coupling of homopropargyl alcohol and aldehydes using Amberlyst-15® as a heterogeneous solid acid.
Keywords
Macrolide; (S)-Zearalenone; Zeranol; Ion-exchange resin; Conjugated ketones; β-Alkoxy conjugated enones
ISSN 2347 – 8853
Indexed in:
 ISSN 2347 – 8853
ISSN 2347 – 8853  Â
Â