
Open Access

Subscription Access
Synthesis, characterization and evaluation of prodrugs of ciprofloxacin clubbed with benzothiazoles through N-Mannich base approach
Mona Piplani, Avtar Chand Rana, Prabodh Chander Sharma
Abstract
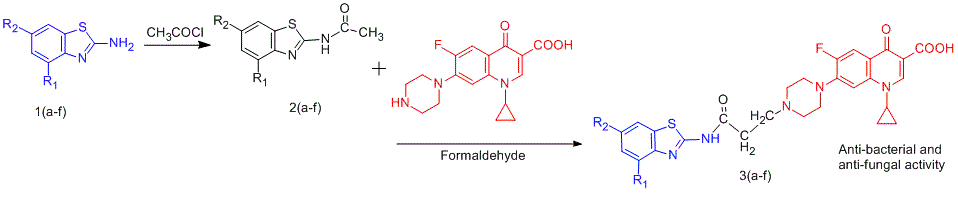
The present study aims towards the design and synthesis of N-Mannich base prodrugs of ciprofloxacin with benzothiazoles to improve the therapeutic potential of antibacterial agent. All of the prodrugs were evaluated for physicochemical characteristics and their structures were confirmed by IR, 1H NMR as well as mass spectroscopy. In vitro dissolution studies of these synthesized prodrugs were carried out in physiological solutions (pH 1.2 and 7.4) resembling gastric and intestinal tract to estimate their hydrolysis. The prodrugs were found to possess high partition coefficient compared to ciprofloxacin. Prodrug C3 has been proved most potent antibacterial agent having MIC value of 12.5 and 25 µg/ml against S. aureus MTCC 96 and S. pyogenus MTCC 442 respectively when compared with ciprofloxacin (MIC value 50 µg/ml). Prodrugs C5 and C6 exhibited comparable antibacterial profile against selected bacterial strains to that of reference drugs. Some of the synthesized prodrugs showed good antifungal activity against selected strains compared to standard drugs.
Keywords
Antibacterial; Prodrug; Ciprofloxacin; Hydrophobicity; Hydrolysis.
Full Text:
PDF

ISSN 2347–9825
Authors/visitors are advised to use Firefox browser for better experience of journal site.
Open Access: Researcher from developing/low economy countries can access the jorunal contents through WHO-HINARI .
 ISSN 2347-9825
ISSN 2347-9825

