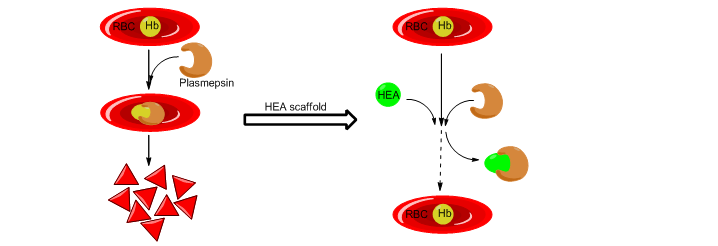
Tetrahedral Hydroxyethylamine: A Privileged Scaffold in Development of Antimalarial Agents
Abstract
Keywords
References
B.R. Stockwell. Exploring biology with small organic molecules. Nature 2004, 432, 846-54.
T.S. Haque, A.G. Skillman, C.E. Lee, H. Habashita, I.Y. Gluzman, T.J. Ewing, D.E. Goldberg, I.D. Kuntz, J.A. Ellman. Potent, low-molecular-weight non-peptide inhibitors of malarial aspartyl protease plasmepsin II. J. Med. Chem. 1999, 42, 1428-40.
D. Nöteberg, E. Hamelink, J. Hultén, M. Wahlgren, L. Vrang, B. Samuelsson, A. Hallberg. Design and Synthesis of Plasmepsin I and Plasmepsin II Inhibitors with Activity in Plasmodium f alciparum-Infected Cultured Human Erythrocytes. J. Med. Chem. 2003, 46, 734-46.
D. Nöteberg, W. Schaal, E. Hamelink, L. Vrang, M. Larhed. High-speed optimization of inhibitors of the malarial proteases plasmepsin I and II. J. Comb. Chem. 2003, 5, 456-64.
D. Muthas, D. Nöteberg, Y.A. Sabnis, E. Hamelink, L. Vrang, B. Samuelsson, A. Karlén, A. Hallberg. Synthesis, biological evaluation, and modeling studies of inhibitors aimed at the malarial proteases plasmepsins I and II. Bioorg. Med. Chem. 2005, 13, 5371-90.
C.-L. Ciana, R. Siegrist, H. Aissaoui, L. Marx, S. Racine, S. Meyer, C. Binkert, R. De Kanteret. al. Novel in vivo active anti-malarials based on a hydroxy-ethyl-amine scaffold. Bioorg. Med. Chem. Lett. 2013, 23, 658-62.
K. Jaudzems, K. Tars, G. Maurops, N. Ivdra, M. Otikovs, J. Leitans, I. Kanepe-Lapsa, I. Domracevaet. al. Plasmepsin Inhibitory Activity and Structure-Guided Optimization of a Potent Hydroxyethylamine-Based Antimalarial Hit. ACS Med. Chem. Lett. 2014, 5, 373-77.
M. Conceicao de Souza, T. Goncalves-Silva, M. Moreth, C. RB Gomes, C. Roland Kaiser, M.d.O.H. das Gracas, M. VN de Souza. Synthesis and In Vivo Antimalarial Evaluation of Novel Hydroxyethylamine Derivatives. Med. Chem. 2012, 8, 266-72.
W. Cunico, M.d.L.G. Ferreira, T.G. Ferreira, C. Penido, M.G. Henriques, L.G. Krettli, F.P. Varotti, A.U. Krettli. Synthesis and antimalarial activity of novel hydroxyethylamines, potential aspartyl protease inhibitors. Lett. Drug Des. Discov. 2008, 5, 178-81.
D. Gupta, R.S. Yedidi, S. Varghese, L.C. Kovari, P.M. Woster. Mechanism-based inhibitors of the aspartyl protease plasmepsin II as potential antimalarial agents. J. Med. Chem. 2010, 53, 4234-47.
V. Aureggi, V. Ehmke, J. Wieland, W.B. Schweizer, B. Bernet, D. Bur, S. Meyer, M. Rottmannet. al. Potent Inhibitors of Malarial Aspartic Proteases, the Plasmepsins, by Hydroformylation of Substituted 7â€Azanorbornenes. Chem. Eur. J. 2013, 19, 155-64.
P. Bhaumik, A. Gustchina, A. Wlodawer. Structural studies of vacuolar plasmepsins. BBA-Proteins Proteom. 2012, 1824, 207-23.
T. Miura, K. Hidaka, Y. Azai, K. Kashimoto, Y. Kawasaki, S.E. Chen, R.F. de Freitas, E. Freire, Y. Kiso. Optimization of plasmepsin inhibitor by focusing on similar structural feature with chloroquine to avoid drug-resistant mechanism of Plasmodium falciparum. Bioorg. Med. Chem. Lett. 2014, 24, 1698-701.
M. Chugh, V. Sundararaman, S. Kumar, V.S. Reddy, W.A. Siddiqui, K.D. Stuart, P. Malhotra. Protein complex directs hemoglobin-to-hemozoin formation in Plasmodium falciparum. Proc. Natl. Acad. Sci. 2013, 110, 5392-97.
J. Liu, E.S. Istvan, I.Y. Gluzman, J. Gross, D.E. Goldberg. Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc. Natl. Acad. Sci. 2006, 103, 8840-45.
A. Silva, A. Lee, S. Gulnik, P. Maier, J. Collins, T. Bhat, P. Collins, R. Cachauet. al. Structure and inhibition of plasmepsin II, a hemoglobin-degrading enzyme from Plasmodium falciparum. Proc. Natl. Acad. Sci. 1996, 93, 10034-39.
R. Wolfenden. Analog approaches to the structure of the transition state in enzyme reactions. Acc. Chem. Res. 1972, 5, 10-18.
G.E. Lienhard. Enzymatic catalysis and transition-state theory. Science 1973, 180, 149-54.
W. Cunico, C.R. Gomes, M. Moreth, D.P. Manhanini, I.H. Figueiredo, C. Penido, M.G. Henriques, F.P. Varotti, A.U. Krettli. Synthesis and antimalarial activity of hydroxyethylpiperazine derivatives. Eur. J. Med. Chem. 2009, 44, 1363-8.
ISSN 2347–9825
Authors/visitors are advised to use Firefox browser for better experience of journal site.
Open Access: Researcher from developing/low economy countries can access the jorunal contents through WHO-HINARI .
 ISSN 2347-9825
ISSN 2347-9825

