Synthetic studies on biologically novel pyrimidinones and related heterocyclic compounds
Abstract
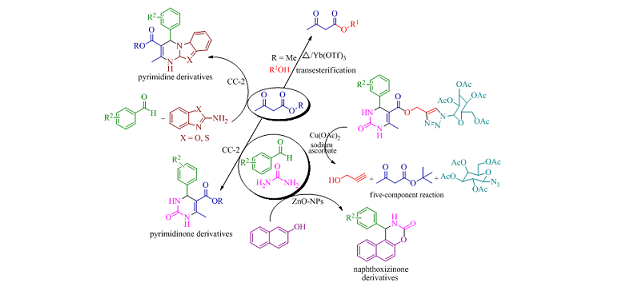
The thesis is constructed in four different parts. The first part describes the Yb(OTf)3 catalyzed/greener protocol for the synthesis of β-ketoesters  via transesterification reaction and its applications. The second part deals with  synthesis of pyrimidinones and pyrimidines derivatives using N-halo reagent [N,N'-dichlorobis(2,4,6-trichlorophenyl)urea] via Biginelli reaction. A five-component reaction involving transesterification/Biginelli/click reaction for the construction of glycoside annulated dihydropyrimidinone derivatives with 1,2,3-triazole linkage analogues was discussed in third part. Finally the fourth fraction engages with ZnO nanoparticles as a heterogeneous catalyst for the synthesis of naphthoxazinone derivatives through Biginelli like reaction.
Keywords
Biginelli Reacion; Pyrimidinones; Pyrimidines; Transesterificaion; β-Ketoesters; ZnO-Nanoparticles; Naphthoxazinones; Click Chemistry
Full Text:
PDFISSN 2347 – 8853
Indexed in:
 ISSN 2347 – 8853
ISSN 2347 – 8853  Â
Â