Synthesis and evaluation of novel hydroxamic acids as potent antibacterial and antifungal agents
Abstract
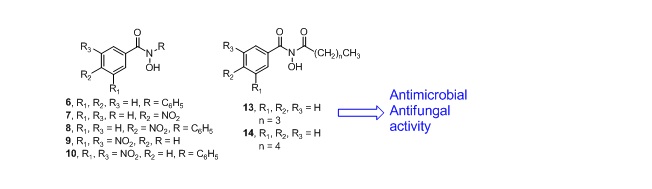
Novel hydroxamic acids (6-10 and 13, 14) were synthesized by reaction of substituted benzoyl chorides (1-3) with substituted hydroxylamine hydrochlorides (4, 5) in presence of aqueous potassium carbonate in diethyl ether at 00C temperature. The new products were purified by silica gel column chromatography and characterized by infrared (IR), proton nuclear magnetic resonance (1H NMR) spectroscopic data, and elemental analysis. All the compounds were evaluated for their antibacterial and antifungal potential by the cup-plate method. From the antibacterial screening it was observed that the compounds, 6, 9, 13 and 14 shows good antibacterial activity against Staphylococcus aureus (zone of inhibition,10-15 mm) as compared to standard streptomycin (zone of inhibition, 18 mm), and Escherichia coli (zone of inhibition,14-16 mm) as compared to streptomycin (zone of inhibition, 22 mm). Fungicidal screening data also revealed that compounds, 6, 7, 8, 9 and 14 imparted maximum activity against Aspergillus niger (zone of inhibition, 10-15 mm) as compared to standard griesofulvin (zone of inhibition, 17 mm), whereas compounds 7, 8, 9, 13 and 14 showed moderate activity against Candida albicans (zone of inhibition, 10-15 mm) as compared to griesofulvin (zone of inhibition, 20 mm).
Keywords
References
A.H. Blatt, Organic Synthesis. Collective; John Wiley and Sons: New York, NY, USA, 1963.
J.M. Altenburger, C. Mioskowski, H. d'Orchymont, D. Schirlin, C. Schalk and C. Tarnus. Useful hydroxylamine derivatives for the synthesis of hydroxamic acids. Tetrahedron Lett. 1992, 33, 5055-5061.
M.A. Staszak, C.W. Doecke. The use of N,O-bis(tert-butoxycarbonyl)-hydroxylamine in the synthesis of N-hydroxylamines and hydroxamic acids. Tetrahedron Lett. 1994, 35, 6021-6024.
V. Krchnák, Mini-Rev. Med. Chem. 2006,6, 27-36.
J. Alsina, F. Albericio, Biopolymers 2003, 71, 454-477.
J. J. Chen, A. F. Spatola. Solid phase synthesis of peptide hydroxamic acids. Tetrahedron Lett. 1997, 38, 1511-1514.
K. Ngu, D. V. Patel, J. Org. Chem. 1997, 62, 7088-7089.
C. D. Floyd, C. N. Lewis, S. R. Patel, M. Whittaker. A method for the synthesis of hydroxamic acids on solid phase. Tetrahedron Lett. 1996, 37, 8045-8048.
L. S. Richter, M. C. Dessai. A TFA-cleavable linkage for solid-phase synthesis of hydroxamic acids. Tetrahedron Lett. 1997, 38, 321-322.
M. F. Gordeev, H. C. Hui, E. M. Gordon, D. V. Patel. A general and efficient solid phase synthesis of quinazoline-2,4-diones. Tetrahedron Lett. 1997, 38, 1729-1732.
B. Barlaam, P. Koza, J. Berriot, Tetrahedron 1999, 55, 7221-7232.
S. L. Mellor, C. McGuire, W. C. Chan. N-Fmoc-aminooxy-2-chlorotrityl polystyrene resin: A facile solid-phase methodology for the synthesis of hydroxamic acids. Tetrahedron Lett. 1997, 38, 3311-3314.
U. Bauer, W. B. Ho, A. M. P. Koskinen. A novel linkage for the solid-phase synthesis of hydroxamic acids. Tetrahedron Lett. 1997, 38, 7233-7236.
N. S. Nandurkar, R. Petersen, K. Qvortrup, V. V. Komnatnyy, K. M. Taveras, S. T. Le Quement, R. Frauenlob, M. Givskov, T. E. Nielsen. A convenient procedure for the solid-phase synthesis of hydroxamic acids on PEGA resins. Tetrahedron Lett. 2011, 52, 7121-7124.
M. Z. Koncic, M. Barbaric, I. Perkovic, B. Zorc. Molecules, 2011, 16, 6232-6242.
R. Singh, Geetanjali, N. Sharma. Monoamine Oxidase Inhibitors for Neurological Disorders: A review. Chem. Biol. Lett., 2014, 1(1), 33-39.
D. K. Pal, S. Saha, J. Adv. Pharm. Tech. Res. 2012, 3, 92-99.
L. P. Tardibono Jr, M. J. Miller, Org. Lett. 2009, 11, 1575-1578.
H. Jahangirian, J. Haron, S. Silong, N. A. Yusof, K. Shameli, S. Eissazadeh, R. R. Moghaddam, B. Mahdavi, M. Jafarzade, J. Med. P. Res. 2011, 5, 4826-4831.
R. B. Hector, W. Lazo, J. Agric. Food Chem. 1996, 44, 1569-1571.
E. Nakagawa, T. Amano, N. Hirai, H. Iwamura, Phytochemistry 1995, 38, 1349-1354.
H. S. Rho, H. S. Baek, S. M. Ahn, J. W. Yoo, D. H. Kim, H. G. Kim, Bull. Korean Chem. Soc. 2009, 30, 475-482.
K. P. Holland, H. L. Elford, V. Bracchi, C. G. Annis, S. M. Schuster, D. Chakrabarti, Antimicrob. Agent. Chemother. 1998, 42, 2456-2458.
S. M. Dankwardt, R. L. Martin, C .S. Chan, H. E. Van Wart, K. A. M. Walker, N. G. Delaet, L. A. Robinson. Arylsulphonyl hydroxamic acids: potent and selective matrix metalloproteinase inhibitors. Bioorg. Med. Chem. Lett. 2001, II, 1465-1468.
Y. K. Agrawal, H. Kaur. React. Funct. Polym. 1999, 39, 155-164.
Y. K. Agrawal, S. A. Patel. Rev. Anal. Chem. 1980, 4, 237-276.
P.N. Reddy, P. Padmaja. Int. Arch. Sci. Tech., 2014, 13(1), 14-18.
F. Huguet, A. Melet, R. A. de Sousa, A. Lieutaud, J. Chevalier, L. Maigre, P. Deschamps, A. Tomas, N. Leulliot, J. M. Pages, I. Artaud. Chem.Med.Chem. 2012, 7, 1020-1030.
C. Lee, E. Choi, M. Cho, B. Lee, S. J. Oh, S. K. Park, K. Lee, H. M. Kim, G. Han. Structure and property based design, synthesis and biological evaluation of γ-lactam based HDAC inhibitors: Part II. Bioorg. Med. Chem. Lett. 2012, 22, 4189-4192.
M. A. Santos, S. M. Marques, T. Tuccinardi, P. Carelli, L. Panelli, A. Rossello, Bioorg. Med. Chem. 2006, 14, 7539-7550.
J. I. Levin. Curr. Top. Med. Chem. 2004, 4, 1289-1310.
V. M. Richon, Y. Webb, R. Merger, T. Sheppard, B. Jursic, L. Ngo, F. Civoli, R. Breslow, R. A. Rifkind, P. A. Marks. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 5705-5708.
A. Mai, M. Esposito, G. Sbardella, S. Massa. Org. Prep. Proced. Int. 2001, 33, 391-394.
H. Bickel, R. Bosshardt, E. Gäumann, P. Reusser, E. Vischer, W. Voser, A. Wettstein, H. Zähner. Helv. Chim. Acta. 1960, 43, 2118-2128.
E. A. Fischer, D. R. McLachlan, T. P. A. Kruck and R. A. Mustard. Development of an Intravenous Desferrioxamine Mesylate Treatment Protocol for Swine: Monitoring of Desferrioxamine and Metabolites by High-Performance Liquid Chromatography. Pharmacology 1990, 41, 263-271.
J. B. Porter, R. Rafique, S. Srichairatanakool, B. A. Davis, F. T. Shah, T. Hair, P. Evans. Recent insights into interactions of deferoxamine with cellular and plasma iron pools: Implications for clinical use. Ann. N.Y. Acad. Sci. 2005, 1054, 155-168.
S. Mahendra, K. Rajesh, B. Bhushan and D. Rajendra. Microwave Assisted Synthesis and Antimicrobial Screening of Pyrazoline-5-ones. Asian J. Chem. 2007, 19, 449-453.
Indian Pharmacopoeia, Ministry of Health and Family Welfare, New Delhi, 1996 A-114.
R. Kumar, R. Johar, A.K. Aggarwal. Tailoring methodologies for the architecture of organometallic frameworks of Bi(V) derived from antibiotics: Spectral, MS, XRPD and molecular modeling with antifungal effectiveness. J. Integr. Sci. Technol. 2013, 1(1), 54-64.
ISSN 2348 – 1889
 |
 ISSN 2348 – 1889
ISSN 2348 – 1889