Removal of Cu(II) from aqueous solution using dead biomass of Bacillus Subtilis
Abstract
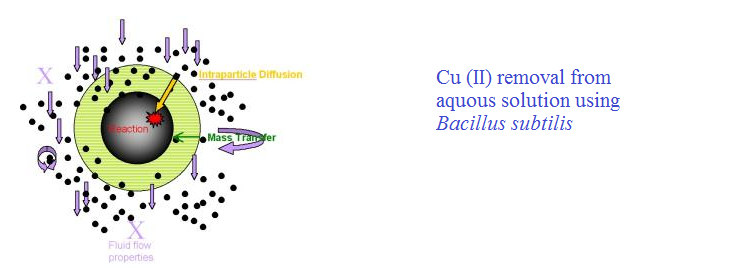
The dead biomass of Bacillus subtilis has been used for the removal of Cu (II) from an aqueous solution. The effects of various parameters such as contact time, adsorbate concentration, pH of the medium and temperature were examined. Optimum removal at 200C was found to be 98.6 % at pH 6.5, with an initial Cu (II) concentration of 100 mgL-1. Dynamics of the sorption process and mass transfer of Cu (II) to Bacillus subtilis were investigated and the values of rate constant of adsorption, rate constant of intraparticle diffusion and the mass transfer coefficients were calculated. Different thermodynamic parameters viz., changes in standard free energy, enthalpy and entropy were evaluated and it was found that the reaction was spontaneous and exothermic in nature. The adsorption data fitted the Langmuir isotherm. A generalized empirical model was proposed for the kinetics at different initial concentrations. The data were subjected to multiple regression analysis and a model was developed to predict the removal of Cu (II) from an aqueous solution.
Keywords
Adsorption; monolayer; Cu (II); Bacillus subtilis; Exothermic; multiple
 ISSN 2321-4635
ISSN 2321-4635