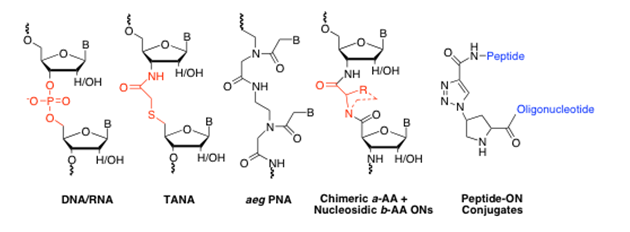
Synthesis and biophysical studies of PNA and chimeric PNA-DNA antisense oligomers with five atom linkages
Abstract
Keywords
Full Text:
PDFReferences
E. Uhlmann, A. Peyman. Antisense oligonucleotides: a new therapeutic principle. Chem.Rev., 1990, 90, 543-584.
S. M. Freier, K. -H. Altmann.The ups and downs of nucleic acid duplex stability: structure-stability studies on chemically-modified DNA:RNA duplexes. Nucleic Acids Res., 1997, 25, 4429-4443.
J. Kurreck. Antisense technologies. Improvement through novel chemical modifications.Eur. J. Biochem.,2003, 270, 1628-1644
V.A. Kumar, K. N. Ganesh. Structure-Editing of Nucleic Acids for Selective Targeting of RNA. Curr. Topics in Med Chem., 2007, 7, 715-726.
J. J. Turner, M. Fabani, A. A. Arzumanov, G. Ivanova, M. J. Gait. Targeting the HIV-1 RNA leader sequence with synthetic oligonucleotides and siRNA: chemistry and cell delivery. Biochim. Biophys. Acta.,2006, 1758, 290-300.
P. C. Zamecnik, M. L. Stephenson. Inhibition of Rous sarcoma virusreplication and cell transformation by a specific oligonucleotide.Proc. Natl. Acad.Sci. U.S.A., 1978, 75, 280-284.
Z. Dominski, R. Kole. Restoration of correct splicing in thalassemic pre-mRNA by antisense oligonucleotides.Proc. Nat. Acad. Sci U.S.A.,1993, 90, 8673-8677
C. J. Wilds, G. Minasov, F. Natt, P. Matt von, K.-H. Altmann, M. Egli. Studies of a chemically modified oligonucleotide containing a 5-atom backbone which exhibits improved binding to RNA. Nucleosides Nucleotides Nucleic Acids, 2001, 20, 991-994.
P.S. Pallan, P. Matt von, C. Wilds, K.-H. Altmann, M. Egli. RNA binding affinities and crystal structure of oligonucleotides containing five-atom amide-based backbone structures.Biochemistry, 2006, 45, 8048-8057.
T. Govindaraju, V. A. Kumar. Backbone-extended pyrrolidine peptide nucleic acids (bepPNA): design, synthesis and DNA/RNA binding studies. Chem. Commun.,2005, 495- 497
K. Gogoi, A. D. Gunjal, V. A. Kumar. Sugar-thioacetamide backbone in oligodeoxyribonucleosides for specific recognition of nucleic acids.Chem Comm.,2006, 2373-2375
R. B. Merrifield. Solid phase synthesis. I. The synthesis of a tetrapeptide. J. Am.Chem. Soc., 1963, 85, 2149-2154.
P. Job. Formation and stability of inorganic complexes in solution.Ann. Chim.,1928, 9, 113-203
K. Gogoi, A. D. Gunjal, U. D. Phalgune, V. A. Kumar. Synthesis and RNA binding selectivity of oligonucleotides modified with five-atom thioacetamido nucleic acid backbone structures. Org. Lett.,2007, 9, 2697-2700.
K. J. Divakar, C. B. Reese.4-(1,2,4-Triazol-1-yl)- and 4-(3-nitro-1,2,4-triazol-1-yl)-1-(ï¢-D-2,3,5-triacetylarabinofuranosyl)pyrimidin-2(1H)-ones. Valuable intermediates in the synthesis of derivatives of 1-(ï¢-D-arabinofuranosyl)cytosine (ara-C). J. Chem. Soc. Perkin Trans., 1982, 1, 1171-1176.
C. Altona, M. Sundaralmgam. Conformational analysis of the sugar ring in nucleosides and nucleotides.New description using the concept of pseudorotation.J. Am. Chem.Soc.,1972, 94, 8205-8212.
J. van Wijk, C. A. G. Haasnoot, F. A. A. M. de Leeuw, B. D. Huckriede, A. WestraHoekzema, C. Altona. PSEUROT 6.2, 1993, PSEUROT 6.3, 1999.Leiden Institute of Chemistry, Leiden University.
L. J. Rinkel, C. Altona. Conformational analysis of the deoxyribofuranose ring in DNA by means of sums of proton-proton coupling constants: a graphical method. J. Biomol. Struct. Dyn.,1987, 4, 621- 649.
K. Gogoi, V. A. Kumar. Chimeric (α-amino acid + nucleoside-β-amino acid)npeptide oligomers show sequence specific DNA/RNA recognition. Chem Comm., 2008, 706-708.
A. De Mico, R. Margarita, L. Parlanti, A. Vescovi, G. Piancatelli. A versatile and highly selective hypervalent iodine (III)/2,2,6,6-tetramethyl-1-piperidinyloxyl-mediated oxidation of alcohols to carbonyl compounds. J. Org. Chem.,1997, 62, 6974-6977.
K. Gogoi, M.V. Mane, S. S. Kunte, V. A. Kumar. A versatile method for the preparation of conjugates of peptides with DNA/PNA/analogue by employing chemo-selective click reaction in water.Nucleic Acids Research, 2007, 35(21):e 139.
M. Manoharan. Oligonucleotide conjugates as potential antisense drugs with improved uptake, biodistribution, targeted delivery, and mechanism of action. AntisenseNucleic Acid Drug Dev., 2002, 12, 103-128.
P. Lundberg, U. Langel. A brief introduction to cell-penetrating peptides.J. Mol. Recogn.,2003,16, 227-233.
N. Venkatesan, B. H. Kim. Peptide Conjugates of Oligonucleotides: Synthesis and Applications. Chem. Rev., 2006, 106, 3712-3761.
H.C. Kolb, M. G. Finn, K. B.Sharpless. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed., 2001, 40, 2004-2021.
ISSN 2347 – 8853
Indexed in:
 ISSN 2347 – 8853
ISSN 2347 – 8853  Â
Â