
Open Access

Subscription Access
Antimycobacterial activity of Schiff’s Bases synthesized from substituted Benzaldehyde active against Mycobacterium
Deeksha Sharma, Niharika Sinha, Anjana Sarkar, Rajesh Kumar Gupta
Abstract
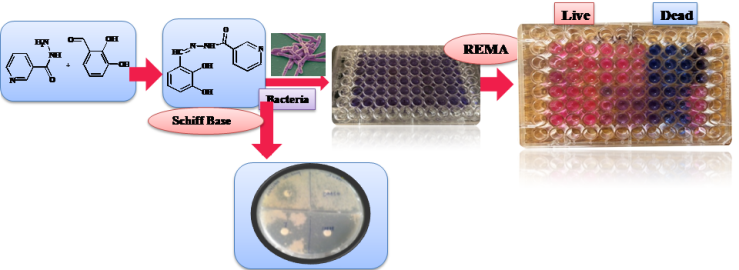
In a preliminary screening we found that few substituted benzaldehyde have growth inhibitory effect against Mycobacterium. Study was planned to convert these substituted benzaldehyde to antimycobacterial Schiff’s bases. Schiff’s bases were synthesized by condensation of 2-Amino pyridine/ its derivative or Thiophene/ Furoic/ Nicotinic acid hydrazide with these substituted benzaldehydes. Compounds were purified and characterized by IR, 1H and 13C-NMR and were subjected to Antimycobacterial testing in two of the Mycobacterial strains; fast growing Mycobacterium smegmatis by disc diffusion method and further in to slow growing Mycobacterium bovis BCG strain using quantitative resazurin microplate assay. Schiff’s bases 3c, 3f and 8a have shown 60, 40 and 50 mm zone of inhibition in M. smegmatis, compared to control, Isoniazid showed 40 mm zone of inhibition. In Mycobacterium bovis, compounds 3c, 3f and 8a showed 53.69, 49.41, and 46.49 % of cell killing at 500 µM, the activity was concentration dependent and reduced viability of 29.73, 37.75 and 36.15% were reported at 125 µM. Isoniazid has shown 60% cell killing at 40 µg/ml. Schiff’s bases prepared from 4-hydroxy or 4-Nitro benzaldehyde with 2-amino pyridine (3c and 3f) and 2, 3 Dihydroxy benzaldehyde with nicotinic acid hydrazide (8a) have shown promising antimycobacterial potential.
Keywords
Anti-TB; substituted benzaldehydes; Mycobacterium smegmatis; Mycobacterium bovis; Schiff’s bases; Resazurin Microtitre plate Assay
Full Text:
PDF

ISSN 2347–9825
Authors/visitors are advised to use Firefox browser for better experience of journal site.
Open Access: Researcher from developing/low economy countries can access the jorunal contents through WHO-HINARI .
 ISSN 2347-9825
ISSN 2347-9825

