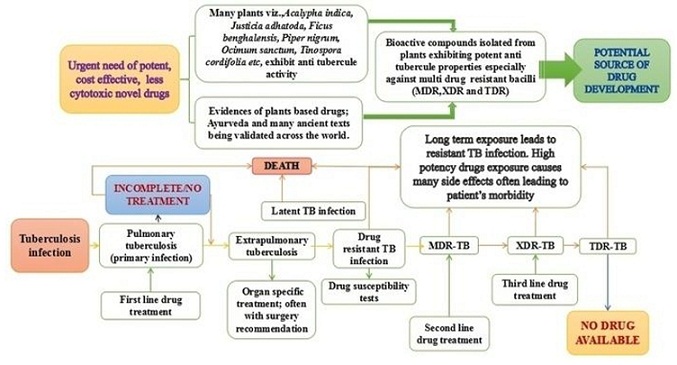
Trials and tribulations in tuberculosis research: Can plant based drug(s) be the solution?
Abstract
Keywords
References
S. Issar. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clinical Microbiol. Rev., 2003, 463–496.
E.W. Tiemersma. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV-negative patients: A systematic review. PLoS ONE, 2011, 6(4), e17601.
M. Uplekar, D. Weil, K. Lonnroth, et al. WHO’s new End TB Strategy. Lancet. 2015, 385, 1799–801.
P.C. Hopewell. Overview of clinical tuberculosis, Tuberculosis: pathogenesis, protection, and control. American Society for Microbiol, B. R. Bloom, Washington, D.C 1994, 25-46.
Global TB report 2015; Available at www.who.int/tb/advisory_bodies/impact_measurement_taskforce, accessed on 06/03/2015.
Global TB report 2015; Available at www.who.int/tb/publications/standardsandbenchmarks/en/, accessed on 06/03/2015
K. Styblo. Overview and Epidemiologic Assessment of the Current Global Tuberculosis Situation with an Emphasis on Control in Developing Countries, Clinical Infect. Diseases, 1989, l(11), S339-S346
R.J. Hobson , A.J. McBride, K.E. Kempsell, J.W. Dale. Use of an arrayed promoter-probe library for the identification of macrophage-regulated genes in Mycobacterium tuberculosis. Microbiol.2002 148,1571-1579
V Arya. A review on anti-tubercular plants, Int J Phar Tech Res, 2011, 3, 872-880.
B. Patwardhan, ADB Vaidya, M Chorghade. Ayurveda and natural products drugdiscovery, Current science 2004, 86(6), 789-799.
P. Chen, R.E. Ruiz, Q. Li, R. F. Silver, W.R. Bishai. Construction and characterization of a Mycobacterium tuberculosis mutant lacking the alternate sigma factor gene, sigma F. Infect. Immun.2000, 68, 5575–5580.
F.M. Collins, Tuberculosis research in a cold climate. Tubercle Lung Dis. 1998, 78, 99–107.
R. Dubos, J. Dubos. The white plague; Little, Brown and Co, Mass. 1952, Boston.
S.K. Hoiseth, B.A. Stocker. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nat Rev 1981, 291, 238.
M. K.Hondalus, S. Bardarov, R. Russell, J. Chan, W. R. Jacobs, Jr., and B. R. Bloom. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect. Immun. 2000. 68, 2888–2898.
M. Iseman, Evolution of drug resistant tuberculosis: a tale of two species. Proc. Natl. Acad. Sci. 1994, 91, 2428-2429.
D.K. Dalton, L. Haynes, C.Q. Chu, S.L. Swain, S. Wittmer. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J. Exp. Med. 2000, 192,117-122.
WHO Global Tuberculosis report 2016 available at www.who.int/tb/publications/global_report/en/ accessed on 27/12/2016
M.A. Horwitz, G. Harth, B.J. Dillon, and S. Maslesa-Galic. Recombinant bacillus Calmette-Guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model, Proc. Nat. Acad. Sci. 2000, USA 97, 13853–13858.
TR Frieden, TR Sterling, SS Munsiff, et al. Tuberculosis: A clinical review on global trends in tuberculosis, emergence of multidrug resistance, and measures for primary prevention of this disease from a public health perspective, Lancet,2003, 362, 887.
J E. Gomez, J D. McKinney. M. tuberculosis persistence, latency, and drug tolerance, Tuberculosis 2004, 84, 29–44.
J. D.MacMicking, C. Nathan, G. Hom, N. Chartrain, D. S. Fletcher, M. Trumbauer, K. Stevens, Q.-W. Xie, K. Sokol, N. Hutchinson, H. Chen, and J.S. Mudgett. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 1995, 81, 641–650.
C. F. Nathan, J.J.B. Hibbs. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol.1991, 3, 65.
E.H. Noss, R.K. Pai, T.J. Sellati, J.D. Radolf, J. Belisle, D.T. Golenbock, W.H. Boom, C.V. Harding. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 2001 167, 910–918.
P.J. Brennan. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis, Elsevier Tuberculosis 2003 83, 91–97
H.T. Waaler, Tuberculosis and poverty. Int. J. Tubercle Lung Dis. 2002. 6, 745–746.
S.H. Cheon, H.Y. Song, M. Phillips, R. Dietze, J.J. Ellner. A whole blood bactericidal assay for tuberculosis. J. Infect. Dis.2001, 183, 1300–1303.
B.J. Wards, D.M. Collins. Electroporation at elevated temperatures substantially improves transformation frequency of slow-growing mycobacteria. FEMS Microbiol. Lett. 1996, 145, 101–105.
A.G. Goldin, W.T. Baker. Tuberculosis of the female genital tract. J Ky Med Assoc 1985, 83, 75
S.N. Tripathy. Endometrial tuberculosis. J Indian Med Assoc, 1987, 85, 136
K. Mayer. Synergistic Pandemics: Confronting the Global HIV and Tuberculosis Epidemics. Clinical Infect. Dis., 2010, 50, Supplement 3, S67http://cid.oxfordjournals.org/content/50/Supplement_3/â€
“Global Tuberculosis Control 2014†available at www.who.int/tb/publications/global_report/ - See more at: http://www.tbfacts.org/tb-hiv/#sthash.Z9WaCmcB.dpuf , accessed on 19/08/2016
T. Sterling. HIV Infection-Related Tuberculosis: Clinical Manifestations and Treatment. Clinical Infect. Dis., 2010, 50, Supp.3, S223-S230
J. Dinnes, J. Deeks, H. Kunst, A. Gibson, E. Cummins, N. Waugh, F. Drobniewski, A. Lalvani, A systematic review of rapid diagnostic tests for the detection of tuberculosis infection, Health Technol Assess 2007, 11(3).
‘TB Testing & Diagnosis’ August 2016, available at https://www.cdc.gov/tb/publications/factsheets/testing/testingfortb_revised.pdf accessed on 16/09/2016
E.R. Huebner, M. F. Schein and J. B. Bass, Jr.The Tuberculin Skin Test, Clinical Infect.Dis, Vol. 17, 1993, 968-975
“Guidelines for intensified case finding and isoniazid preventative therapy for people living with HIV in resource constrained settingsâ€, Geneva, WHO, 2011, http:/www.who.int/tb/publications/2011/, accessed on 02/05/2015
C. M. Denkinger,K. Dheda,M. Pai. Guidelines on interferon-γ release assays for tuberculosis infection: concordance, discordance or confusion?, Clin Microbiol Infect, 2011, 17, 806–814
TC Kim , RS Blackman, KM Heatwole, T Kim, DF Rochester. Acid-fast bacilli in sputum smears of patients with pulmonary tuberculosis. Prevalence and significance of negative smears pretreatment and positive smears post-treatment. The American Rev. of Resp.Dis. 1984, 129(2), 264-268
D. J Lucian, A.Cattamanchi, L.E. Cuevas, P.C. Hopewell, K.R. Steingart. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: a systematic review and meta-analysis, The Lancet Infect. Dis 2012, 13(2), 147–154
S. Ramaswamy, J.M. Musser, Molecular genetic basis of antimicrobial agent resistance inMycobacterium tuberculosis: 1998 update, Tubercle and Lung Dis., 1998, 79, 3-29
NS1Shah, A Wright, GHBai, LBarrera, F Boulahbal, M NCasabona, FDrobniewski, C Gilpin, M Havelková, RLepe, RLumb, BMetchock, FPortaels, MF Rodrigues, R SGerdes, VADeun, V Vincent, K Laserson, C Wells, JP Cegielski, Worldwide emergence of extensively drug-resistant tuberculosis, Emerg Infect Dis. 2007 (3), 380-7.
HA Ward, DD Marciniuk, VH Hoeppner, T Jones, Treatment outcome of multidrug-resistant tuberculosis among Vietnamese immigrants. Int J Tuberc Lung Dis 2005, 9, 164–9
E Nathanson, C LWeezenbeek, ML Rich, R Gupta, J Bayona, K Blondal, et al. Multidrug-resistant tuberculosis management in resource-limited settings. Emerg Infect Dis 2006, 12, 1389–97
World Health Organization, Fact Sheet No. 104: Tuberculosis, 2007, www.who.int/mediacentre/factsheets/fs104/en/index.html accessed on 04/05/2016
Global Tuberculosis control: survillance, planning and financing. WHO report 2008 Geneva World Helth Organization, 2008, available at www.who.int/tb/dots/planning_budgeting_tool/en/index.html accessed on 04/05/2016
WHO. "Multidrug-resistant tuberculosis (MDR-TB) 2013 Update" (PDF). World Health Organization. 2013, available at: www.who.int/tb/challenges/mdr accessed on 04/05/2016
WHO Global Task Force outlines measures to combat XDR-TB worldwide, World Health Organisation, 2006, available at http://www.who.int/mediacentre/news/notes/2006/np29/en/ accessed on 04/05/2016
B.F. Lima, M. Tavares, Risk factors for extensively drug-resistant tuberculosis: a review. The Clinical Respiratory Journal 2013, 8.1,11-23.
J. Singh, B.S. Chhikara. Comparative global epidemiology of HIV infections and status of current progress in treatment. Chem. Biol. Lett., 2014, 1(1), 14-32.
Centers for Disease Control and Prevention, Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR, 2011, 60(48), 1650-1653.
A. Jindani, et al. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: Int. multicentre randomised trial. Lancet, 2004, 364(9441), 1244-1251.
Clinical trials, Pharmacokinetic Study to Evaluate Anti-mycobacterial Activity of TMC207 in Combination With Background Regimen (BR) of Multidrug Resistant Tuberculosis (MDR-TB) Medications for Treatment of Children/Adolescents Pulmonary MDR-TB, available at https://clinicaltrials.gov/ct2/show/study/NCT02354014, accessed on 26/09/2016,
Clinical trials, TBTC Study 31: Rifapentine-containing Tuberculosis Treatment Shortening Regimens (S31/A5349), available at https://clinicaltrials.gov/ct2/show/record/NCT02410772, accessed on 26/09/2016
Clinical trials, A Multiple Ascending Dose Study With a Dose Formulation Comparison Cohort to Evaluate the Safety, Tolerability, and Pharmacokinetics of TBA-354 in Healthy Adult Subjects , available at https://clinicaltrials.gov/show/NCT02606214, accessed on 26/09/2016
Clinical trials, A Phase 3 Study Assessing the Safety and Efficacy of Bedaquiline Plus PA-824 Plus Linezolid in Subjects With Drug Resistant Pulmonary Tuberculosis, available at https://clinicaltrials.gov/ct2/show/record/NCT02333799, accessed on 26/09/2016
YI Ishizaki, C Hayashi, K Inoue, M Igarashi, Y Takahashi, V Pujari, DJ Crick, PJ Brennan, A NomotoInhibition of the first step in synthesis of the mycobacterial cell wall core, catalyzed by the GlcNAc-1-phosphate transferase WecA, by the novel caprazamycin derivative CPZEN-45, J Biol Chem. 2013, 288(42), 30309-19.
Clinical trials, Evaluation of SQ109, High-dose Rifampicin, and Moxifloxacin in Adults With Smear-positive Pulmonary TB in a MAMS Designavailable at http://www.clinicaltrials.gov/ct2/show/NCT01785186?term=sq-109&rank=6, accessed on 26/09/2016
Clinical trials, Phase 2a EBA Trial of AZD5847, available at http://clinicaltrials.gov/ct2/show/NCT01516203, accessed on 26/09/2016
DI Zhang,Y Lu , K Liu, B Liu, J Wang, G Zhang, H Zhang, Y Liu, B Wang, M Zheng, L Fu, Y Hou,N Gong,Y Lv, C Li, CB Cooper, AM Upton, D Yin, Z Ma, H Huang, Identification of less lipophilic riminophenazine derivatives for the treatment of drug-resistant tuberculosis, J Med Chem. 2012, 55(19), 8409-17.
clinical trials, Early Access of TMC207 in Patients With Extensively Drug Resistant or Pre-XDR Pulmonary Tuberculosis,available at https://clinicaltrials.gov/ct2/show/NCT01464762, accessed on 26/09/2016
clinical trials, Safety and Efficacy Trial of Delamanid for 6 Months in Patients With Multidrug Resistant Tuberculosis, available at https://clinicaltrials.gov/ct2/show/record/NCT01424670, accessed on 26/09/2016
Clinical trials, Safety, Tolerability And Pharmacokinetics Study Of Single Doses Of PNU-100480 In Healthy Adults,available at http://clinicaltrials.gov/ct2/show/NCT00871949, accessed on 26/09/2016
Clinical trials, SQ609 for the Treatment of Tuberculosis, available at http://www.sequella.com/docs/SQ609%20TB%20product%20summary.pdf, accessed on 26/09/2016
B Nikonenko,V M. Reddy, EBogatcheva, M Protopopova, L Einck, and CA. Nacy, Therapeutic Efficacy of SQ641-NE against Mycobacterium tuberculosis, Antimicrob Agents Chemother. 2014, 58(1), 587–589.
ADisratthakit and NDoi, In Vitro Activities of DC-159a, a Novel Fluoroquinolone, against Mycobacterium Species, Antimicrob. Agents Chemother. 2010, 54(6), 2684-2686.
A.A Mungantiwar, and A.S. Phadke, Immunomodulation: Therapeutic Strategy through Ayurveda, Scientific Basis for Ayurvedic Therapies, CRC Press, 2003. L.C 72 ISBN 084931366X
PK Debnath,, J Chattopadhyay, D Ghosal, P Bhattacharya. Immunomodulatory Role of Ayurvedic Rasayan for Quality of Life. International Nat Conf. 1998, 38
K. Ilango, V Chitra, K kanimozhi and R Balaji, J. pharma sci and research. 2009, 1(2),67-73.
J. Srivastava , T. Regan JM Pollok, A. Kruger, Medicinal plant; An expanding role in development. The world Bank, Washington, 1996, 8(6).
R Gupta, B. Thakur, P. Singh, H.B. Singh, V.D. Sharma, V.M. Katoch, S.V.S. Chauhan. Anti-tuberculosis activity of selected medicinal plants against multi-drug resistant Mycobacterium tuberculosis isolates, Indian J Med Res 131, 2010, 809-813
S.S. Semenya, A. Maroyi. Medicinal plants used for the treatment of tuberculosis by bapedi traditional healers in three districts of the Limpopo province, South Africa, Afr J Tradit Complement Altern Med. 2013 10(2), 316-323.
N. Lall, J.J.M. Meyer. In vitro inhibition of drug-resistant and drug-sensitive strains of Mycobacterium tuberculosis by ethnobotanically selected South African plants, Journal of Ethnopharmacol, 1999, 66, 347–354.
T.P. Singh, O.M. Singh, H.B. Singh. Adhatoda vasica Nees: Phytochemical and Pharmacological Profile, The Natural Products Journal, 2011, 1, 29-39.
C.A Devi. Docking study on Mycobacterium tuberculosis receptors AccD5 and PKS18 with selected phytochemicals, IOSR Journal of Pharmacy and Biological Sciences, 2012, 4(3), 1-4.
Pulmonary tuberculosis and its ayurvedic treatment, available at http://blog.ayurvedatreatments.co.in/page/15/ accessed on 03/09/2016
P.K. Debnath,J Chattopadhyay, A Mitra , A Adhikari, M S Alam, SK Bandopadhyay, J Hazra. Adjunct therapy of Ayurvedic medicine with anti tubercular drugs on the therapeutic management of pulmonary tuberculosis, J Ayurveda Integr Med. 2012, 3(3), 141–149.
G.K. Datta, P.K. Debnath. Stress Adaptation in Ayurveda by Immunomodulatory Rasayana in National Seminar on Rasayana Proceedings published by CCRAS, New Delhi. 2001, 60–75.
LK Ojha , HS Bajpai, PV Sharma, MN Khanna, PK Shukla, TN Sharma. Chyawanprash as anabolic agent Experimental Study (preliminary work) J Res Indian Med. 1973, 8, 11–4.
A. Sivakumar, G. Jayaraman. Antituberculosis activity of commonly used medicinal plants of south India. Journal of Medicinal Plants Research 2011, 5(31), 6881-6884.
R Gautam, ASaklani, S M. Jachak. Indian medicinal plants as a source of antimycobacterial agents, J. Ethnopharmac 2007, 110, 200–234.
J. Chottopadhyay Study on the effect of Ayurvedic medicine on the bioavailability of Anti-tubercular drugs, Ph.D. (Ay.) thesis. Kolkata: Univ. of Calcutta, 2006
A Bhatt, RK Singh, R Kant. Synthesis of novel Imidazo [1,2-b] pyridazine derivatives and study of their biomedicinal efficacy, Chem. Biol. Lett. 2016, 3(2), 38-43
Signacimuthu, N. Shanmugam. Antimycobacterial activity of two natural alkaloids, vasicine acetate and 2-acetyl benzylamine, isolated from Indian shrub Adhatoda vasica Ness. Leaves, J. Biosci. 2010, 565–570.
A. Gemechu, M. Giday, A. Worku, G. Ameni. In vitro Anti-mycobacterial activity of selected medicinal plants against Mycobacterium tuberculosis and Mycobacterium bovis Strains, BMC Comp and Alt Med, 2011, 13-291.
R. Kaur, K. Singh, R. Singh. 1,5-Benzothiazepine: Bioactivity and targets, Chem. Biol. Lett. 2016, 3(1), 18-31
R.B.L. Scodroa, C.T.A. Pires , V.S. Carrara, C.O.T. Lemos, L. Cardozo-Filhoe, V.A. Souzaf,A.G. Corrêaf, V.L.D. Siqueiraa, M.V.C. Lonardoni , R.F. Cardoso, D.A.G. Cortez. Anti-tuberculosis neolignans from Piper regnellii, Phytomedicine 2013, 600–604.
A. GarcÃa, V. BGarcÃa, J.P. PNicolás, G. Rivera. Recent advances in antitubercular natural products, Eur. J. Med. Chem 2012, 49, 1–23.
X. Luo, D. Pires, J.A. Aı´nsa, B Gracia, N Duarte, SMulhovo, E Anes, MJose´ U. Ferreira. Zanthoxylum capense constituents with antimycobacterial activity against Mycobacterium tuberculosis in vitro and ex vivo within human macrophages, Journal of Ethnopharmacol 2013, 146, 417–422.
B. Singh, M. Jain, S.V. Singh, K. Dhama, G.K. Aseri, N. Jain, M. Datta, N. Kumar, P. Yadav, S. Jayaraman, S. Gupta, K.K. Chaubey and J. S. Sohal. Plants as Future Source of Anti-Mycobacterial Molecules andArmour for Fighting Drug Resistance, Asian Journal of Animal and Veterinary Advances 2015, 10 (9), 443-460.
P Ranasinghe, S Pigera, GA S Premakumara, P Galappaththy, G R Constantine, P Katulanda. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): a systematic review, BMC Complement Altern Med. 2013, 13, 275.
A.O. Sergio, C.V. Fabiola, N.M. Guadalupe, R.C. Blanca, H.O. León. Evaluation of antimycobacterium activity of the essential oils of cumin (Cuminum cyminum), clove (Eugenia caryophyllata), cinnamon (Cinnamomum verum), laurel(Laurus nobilis) and anis (Pimpinella anisum) against Mycobacterium tuberculosis, Advances in Biological Chemistry, 2013, 3, 480-484
ISSN 2347–9825
Authors/visitors are advised to use Firefox browser for better experience of journal site.
Open Access: Researcher from developing/low economy countries can access the jorunal contents through WHO-HINARI .
 ISSN 2347-9825
ISSN 2347-9825

