Evaluation of inhibition of protein tyrosine phosphatase 1B by calixarene-based α-ketophosphonic acids
Abstract
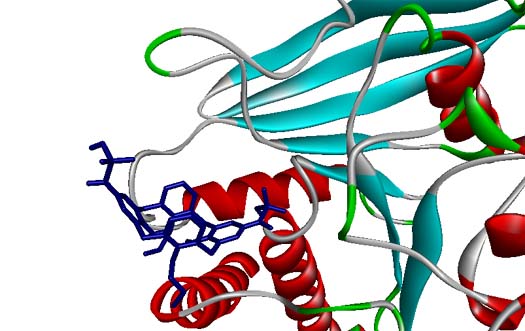
Protein tyrosine phosphatase 1B (PTP1B) is known to be implicated in insulin resistance, and inhibitors of PTP1B were supposed to be useful to regulate insulin and leptin signalling pathways. In this paper, calix[4]arene mono- and bis-α-ketophosphonic acids were tested in vitro as potential inhibitors of PTP1B. The calix[4]arene-based mimics of phosphate esters were synthesized through the reaction of corresponding acyl chlorides with triisopropylphosphite following by dealkylation of α-ketophosphonate diisopropyl ester groups. The inhibiting capacity of the synthesized compounds toward PTP1B was higher than that for other protein tyrosine phosphatases such as TC–PTP, LAR, MEG1, MEG2, and SHP2. The kinetic studies showed that the inhibitors can bind to the active site of PTP1B by replacing the substrate. According to molecular docking results, phosphonate groups of the inhibitors form hydrogen bonds with amino acid residues of P-loop. In addition, macrocyclic platform provides hydrophobic and van der Waals contacts with the enzyme.
Keywords
ISSN 2347–9825
Authors/visitors are advised to use Firefox browser for better experience of journal site.
Open Access: Researcher from developing/low economy countries can access the jorunal contents through WHO-HINARI .
 ISSN 2347-9825
ISSN 2347-9825

