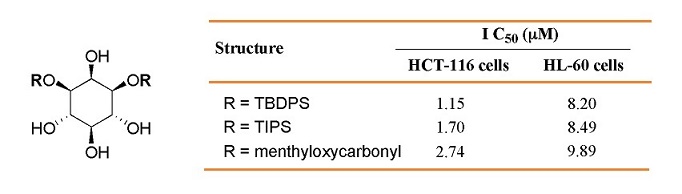
Effects of 1,3-di-O-substituted-myo-inositol derivatives on the antiproliferation and caspase-3 activity of HCT-116 and HL-60 cells
Abstract
Keywords
References
H. Akl, G. Bultynck. Altered Ca(2+) signaling in cancer cells: proto-oncogenes and tumor suppressors targeting IP3 receptors. Biochim. Biophys. Acta. 2013, 1835, 180-193.
T. F. Franke, D. R. Kaplan, L. C. Cantley. PI3K: downstream AKTion blocks apoptosis. Cell 1997, 88, 435-437.
D. Kong, T. Yamori. Phosphatidylinositol 3-kinase inhibitors: promising drug candidates for cancer therapy. Cancer Sci. 2008, 99, 1734-1740.
F. Stauffer, S. M. Maira, P. Furet, C. GarcÃ-EcheverrÃa. Imidazo[4,5-c]quinolines as inhibitors of the PI3K/PKB-pathway. Bioorg. Med. Chem. Lett. 2008, 18, 1027-1030.
S. M. Maira, F. Stauffer, J. Brueggen, P. Furet, C. Schnell, C. Fritsch, S. Brachmann, P. Chène, A. De Pover, K. Schoemaker, D. Fabbro, D. Gabriel, M. Simonen, L. Murphy, P. Finan, W. Sellers, C. GarcÃa-EcheverrÃa. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008, 7, 1851-1863.
J. R. Garlich, P. De, N. Dey, J. D. Su, X. Peng, A. Miller, R. Murali, Y. Lu, G. B. Mills, V. Kundra, H. K. Shu, Q. Peng, D. L. Durden. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008, 68, 206-215.
S. J. Angyal, P. T. Gilham, G. J. Melrose. Cyclitols. XVI. Toluene-p-sulphonyl derivatives of myoinositol. Acetyl migration in anhydrous pyridine solution. J. Chem. Soc. 1965, 5252-5258.
S. K. Chung, Y. T. Chang. Syntheses of myo-inositol-1,2,3,5- and -2,4,5,6-tetrakisphosphates, unusual inhibitors of myo-inositol-1,4,5-triphosphate 3-kinase. Bioorg. Med. Chem. Lett. 1997, 7, 2715-2718.
S. Yamauchi, M. Hayashi, Y. Watanabe. One-Step Regioselective Functionalization of myo-Inositol by Dissolution Strategy. Synlett 2009, 2287-2290.
Y. Watanabe, T. Uemura, S. Yamauchi, K. Tomita, T. Saeki, R. Ishida, M. Hayashi. Regioselective functionalization of unprotected myo-inositol by electrophilic substitution. TETRAHEDRON 2013, 69, 4657-4664.
R. Singh, Geetanjali, N. Sharma. Monoamine Oxidase Inhibitors for Neurogical Disorders: A review. Chem. Biol. Lett. 2014, 1, 33-39.
B. S. Chhikara, A. K. Mishra, V. Tandon. Synthesis of bifunctional chelating agents to label monoclonal antibodies for radioimmunodiagnosis of cancer. Int. Arch. Sci. Technol. 2006, 6, 5-9.
N. Hatae, J. Nakamura, T. Okujima, M. Ishikura, T. Abe, S. Hibino, T. Choshi, C. Okada, H. Yamada, H. Uno, E. Toyota. Effect of the orthoquinone moiety in 9,10-phenanthrenequinone on its ability to induce apoptosis in HCT-116 and HL-60 cells. Bioorg. Med. Chem. Lett. 2013, 23, 4637-4640.
T. Patel, G. J. Gores, S. H. Kaufmann. The role of proteases during apoptosis. FASEB J. 1996, 10, 587-597.
C. Dai, S. B. Krantz. Interferon gamma induces upregulation and activation of caspases 1, 3, and 8 to produce apoptosis in human erythroid progenitor cells. Blood 1999, 93, 3309-3316.
L. C. Cantley, B. G. Neel. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 4240-4245.
G. Kulik, J. P. Carson, T. Vomastek, K. Overman, B. D. Gooch, S. Srinivasula, E. Alnemri, G. Nunez, M. J. Weber. Tumor necrosis factor alpha induces BID cleavage and bypasses antiapoptotic signals in prostate cancer LNCaP cells. Cancer Res. 2001, 61, 2713-2719.
S. Semba, N. Itoh, M. Ito, M. Harada, M. Yamakawa. Down-regulation of PIK3CG, a catalytic subunit of phosphatidylinositol 3-OH kinase, by CpG hypermethylation in human colorectal carcinoma. Clin. Cancer Res. 2002, 8, 1957-1963.
ISSN 2347–9825
Authors/visitors are advised to use Firefox browser for better experience of journal site.
Open Access: Researcher from developing/low economy countries can access the jorunal contents through WHO-HINARI .
 ISSN 2347-9825
ISSN 2347-9825

